What scientific evidence is there that homeopathy works?
The scientific evidence for homeopathy is based on the same types of clinical trials as those used to test conventional medical treatments.
By the end of 2023, 286 randomised controlled trials of homeopathic treatment for 152 medical conditions have been published in peer-reviewed journals with sufficient information provided to analyse the results.
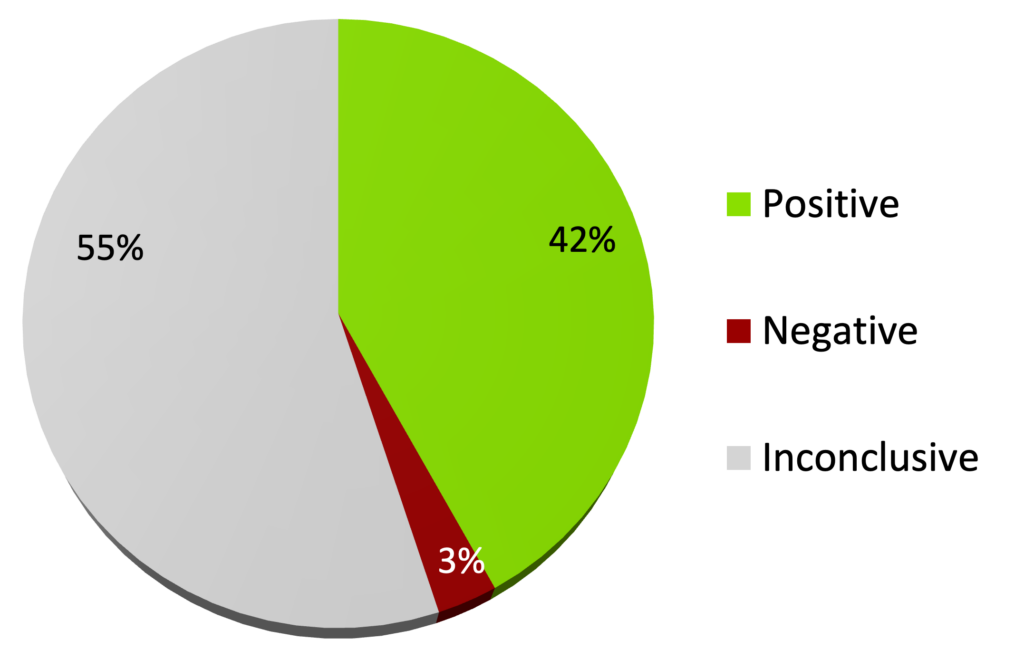
Of these, 166 were double-blind, randomised, placebo-controlled trials, covering 100 different medical conditions1:
- 42% were positive (70 trials) – finding that homeopathy was effective
- 3% were negative (5 trials) – finding that homeopathy was ineffective
- 55% were inconclusive (91 trials)
166 DB-RCTs of homeopathy (end of 2023)
Chart A
Full list of double-blind randomised placebo controlled trials.
Studies excluded: Non-peer-reviewed journals and other non-academic sources; non-human studies; prophylaxis studies; cross-over design; single-blind trials.
How does this compare with evidence for conventional medicine?
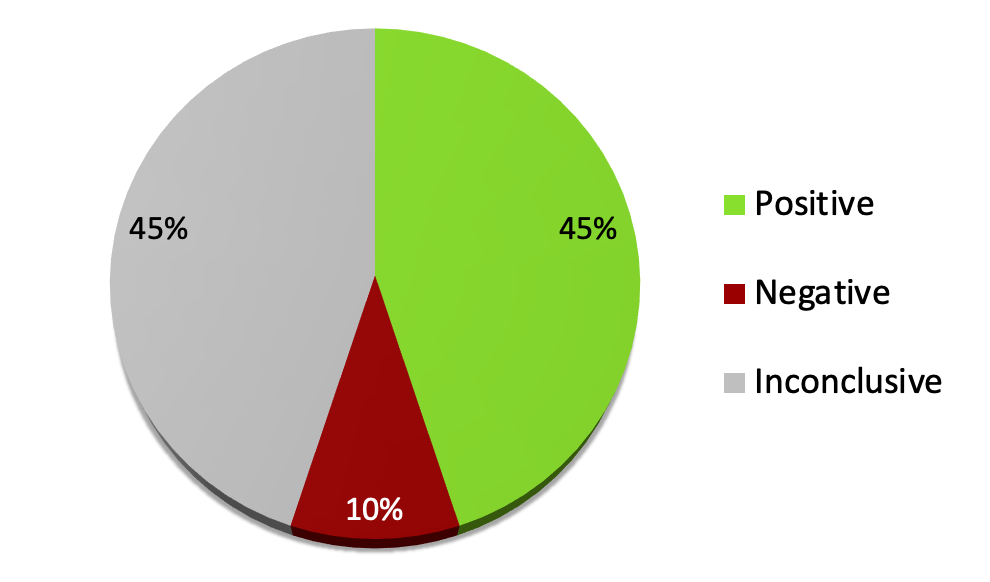
1128 systematic reviews of RCTs of conventional medicine
Chart B
An analysis of 1128 systematic reviews of RCTs of conventional medicine had strikingly similar findings in terms of the proportion of positive clinical trials2:
- 45% were positive – the treatments were likely to be beneficial
- 10% were negative – the treatments were likely to be harmful
- 45% were inconclusive – the evidence did not support either benefit or harm.
Although the overall balance of evidence is broadly similar in homeopathy and conventional medicine, it is important to recognise a vast difference in the quantity of research carried out. Chart A shows analysis of 166 studies out of a total of 286 individual trials on homeopathy. By contrast, chart B shows analysis of 1128 out of over 4000 Cochrane systematic reviews on conventional medicine published by 2011, each analysing multiple individual trials.
This highlights the need for more research in homeopathy, particularly large-scale high quality repetitions of the most promising positive studies.
The difference in quantity is also not surprising when one considers that only a tiny fraction of available funding is allocated to research into ‘complementary and alternative medicine’ (CAM).
For example, an analysis in the UK found that only 0.0085% of the total medical research budget was spent on CAM.3 And in the US, <0.4% of the requested $51.1 billion annual medical budget is allocated to CAM for use by the National Center for Complementary and Integrative Health.4
- Full list of randomised placebo controlled trials in Chart A | Link
- Boas PJFV, Spagnuolo RS, Kamegasawa A, et al. Systematic reviews showed insufficient evidence for clinical practice in 2004: what about in 2011? The next appeal for the evidence-based medicine age. J Eval Clin Pract, 2013; 19(4):633-7 | PubMed
- Lewith GT. Funding for CAM. BMJ., 2007; 335(7627): 951. | PubMed
- The National Institutes of Health (NIH) Office of Budget, states that in 2023 the NIH requested $51.1 billion for the next fiscal year. Funded by NIH, the National Center for Complementary and Integrative Health (NCCIH) enacted budget for 2023 was $170.3 million, of which $89.9 million was used on Research Grants. Thus, <0.4% (0.33% = $170.3 million/$51.1 billion) of the NIH budget was for Integrative Medicine.
For a more in-depth, rigorous analysis of placebo-controlled trials on homeopathy, see Dr Robert Mathie’s programme of systematic reviews here.