Clinical trials overview
Systematic reviews of homeopathy clinical trials
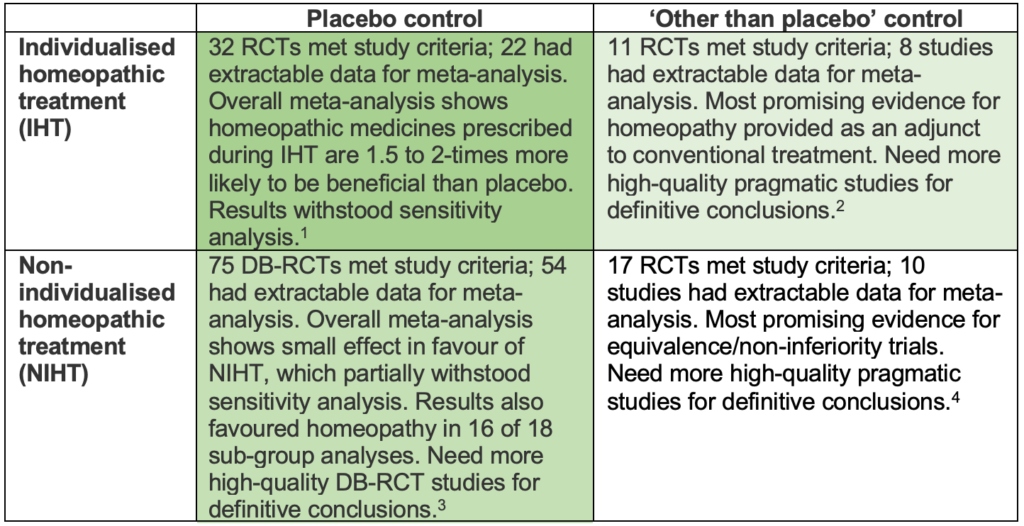
The most robust data on efficacy of homeopathy comes from a 2014 meta-analysis of placebo-controlled double-blind randomised controlled trials which found that homeopathic medicines, when prescribed during individualised treatment, are 1.5- to 2.0-times more likely to have a beneficial effect than placebo.1 This study is one of the four most recent comprehensive systematic reviews conducted by Dr Robert Mathie within a ten-year programme of work.
Historically, global systematic reviews have analysed all clinical trials together, regardless of the type of homeopathic treatment used and medical condition being treated (see reviews 1991-2005).
Dr Mathie’s systematic reviews take us several steps forward in clarifying the evidence base by separating trials by type of treatment (whether each participant received an individualised or a standardised prescription), as well as by comparators (whether the control group was given placebo or something ‘other than placebo’).
Individualised homeopathic treatment is a tailored prescription provided after consultation with a homeopathic practitioner (somewhat similar to a visit to your GP/medical specialist). This is very different from non-individualised homeopathy in which the same homeopathic medicine is used by all patients, usually an over-the-counter medicine bought at a pharmacy containing multiple homeopathic ingredients.
The next step for homeopathy researchers will be to conduct more clinical trials on specific medical conditions such that there is sufficient data to reach definitive conclusions about how effective homeopathy is for individual medical conditions.
When taken collectively, Dr Mathie’s programme of work includes some important positive findings for homeopathy.
Summary of Dr Mathie’s Systematic Review Programme
Individualised homeopathic treatment
Taken together, the two systematic reviews on individualised homeopathic treatment1,2 provide the most rigorous and up-to-date assessment of the key question, ‘Does homeopathy work when prescribed as ‘usual care’ by a trained practitioner?’ The answer is clearly ‘yes’ – the data shows individualised homeopathic medicines to have an effect beyond placebo.
Non-individualised homeopathic treatment
The evidence for non-individualised homeopathy is promising, but less clear. Analysis of 54 placebo-controlled trials showed a small beneficial effect beyond placebo (SMD -0.33; 95% CI -0.44 to -0.21)3 that partially withstood sensitivity analysis and remained positive in 16 of 18 sub-analyses.
Further research is needed to consolidate and expand the current clinical trial evidence base in homeopathy. However, these systematic reviews and meta-analyses have identified areas of most promise, helping researchers know where to focus their efforts in order to inform decision-makers and patients about the potential of homeopathic treatments.
It is worth noting that none of Dr Mathie’s reviews were included in the 2015 NHMRC Homeopathy Review, being published at a later date. However, it is hard to understand why the 2017 EASAC position statement on homeopathy makes no mention of Dr Mathie’s 2014 meta-analysis of individualised homeopathy, being the most relevant and robust data on the topic available at the time.
Robert Mathie presenting his findings on Individualised homeopathy at the HRI Malta 2017 conference:
Historical systematic reviews (1991-2005)
5 global systematic reviews were conducted up to 2005, each conducting a meta-analysis of clinical trials on all types of homeopathic treatment for all medical conditions together.5
- Four studies were positive – suggesting that there was some evidence of an effect of homeopathy beyond placebo, but more high-quality research would be needed to reach definitive conclusions5-8
- One study was negative – concluding that homeopathy had no effect beyond placebo9
All five of these global SRs are now out of date – with the first review by Kleijnen et al. being 31 yrs old5 and Shang et al.9 being 17 yrs old.
- Kleijnen et al. 1991: ‘At the moment the evidence of clinical trials is positive but not sufficient to draw definitive conclusions because most trials are of low methodological quality and because of the unknown role of publication bias. This indicates that there is a legitimate case for further evaluation of homoeopathy, but only by means of well performed trials.’5
- Linde et al. 1997: ‘The results of our meta-analysis are not compatible with the hypothesis that the clinical effects of homeopathy are completely due to placebo. However, we found insufficient evidence from these studies that homeopathy is clearly efficacious for any single clinical condition. Further research on homeopathy is warranted provided it is rigorous and systematic.’ 6
- Linde et al. 1999: ‘We conclude that in the study set investigated, there was clear evidence that studies with better methodological quality tended to yield less positive results.’7
- Cucherat et al. 2000: ‘There is some evidence that homeopathic treatments are more effective than placebo; however, the strength of this evidence is low because of the low methodological quality of the trials. Studies of high methodological quality were more likely to be negative than the lower quality studies. Further high quality studies are needed to confirm these results.’8
- Shang et al. 2005: ‘Biases are present in placebo-controlled trials of both homoeopathy and conventional medicine. When account was taken for these biases in the analysis, there was weak evidence for a specific effect of homoeopathic remedies, but strong evidence for specific effects of conventional interventions. This finding is compatible with the notion that the clinical effects of homoeopathy are placebo effects.’9
It is remarkable that the single negative study, Shang et al. 2005, continues to be cited most widely in anti-homeopathy publications (as recently as the 2017 EASAC Statement) despite only covering data up to 2003, being contradictory to the remainder of evidence in this category and serious concerns regarding its scientific reliability.
Systematic reviews of clinical trials without meta-analysis
The most current overview of clinical trials – the Australian NHMRC report10 – was published in 2015. This study includes individual clinical trials up to 2010 (captured within SRs published up to 3 January 2013) and separates the data by medical condition. The NHMRC report has attracted international criticism for its unprecedented and unscientific methodology. NHMRC is currently under investigation by the Commonwealth Ombudsman to answer charges of scientific misconduct in relation to this report.
- Mathie RT et al. Randomised placebo-controlled trials of individualised homeopathic treatment: systematic review and meta-analysis. Systematic Reviews, 2014; 3: 142 | Full text
- Mathie RT et al. Systematic Review and Meta-Analysis of Randomised, Other-than-Placebo Controlled, Trials of Individualised Homeopathic Treatment. Homeopathy, 2018; 107(4): 229-243 |Full text
- Mathie RT et al. Randomised, double-blind, placebo-controlled trials of non-individualised homeopathic treatment: systematic review and meta-analysis. Systematic Reviews, 2017; 6(1):63 | Full text
- Mathie RT et al.Systematic Review and Meta-Analysis of Randomised, Other-than-Placebo Controlled, Trials of Non-Individualised Homeopathic Treatment. Homeopathy, 2019; eFirst | Abstract
- Kleijnen, J., Knipschild, P. & ter Riet, G. Clinical trials of homeopathy. BMJ, 1991; 302: 960 | PubMed
- Linde, K. et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials. Lancet, 1997; 350:834–843 | PubMed
- Linde, K. et al. Impact of study quality on outcome in placebo-controlled trials of homeopathy. J. Clin. Epidemiol., 1999; 52: 631–636 | PubMed
- Cucherat, M., Haugh, M. C., Gooch, M. & Boissel, J. P. Evidence of clinical efficacy of homeopathy. A meta-analysis of clinical trials. HMRAG. Homeopathic Medicines Research Advisory Group. Eur. J. Clin. Pharmacol., 2000; 56: 27–33 | PubMed
- Shang A, Huwiler-Muntener K, Nartey L, et al. Are the clinical effects of homeopathy placebo effects? Comparative study of placebo-controlled trials of homeopathy and allopathy. Lancet, 2005; 366: 726–732 | PubMed
- Australian National Health and Medical Research Council (NHMRC) 2015 Homeopathy Review | Link





